Traditionally, this process requires high temperatures above 300°C, making it energy-intensive and costly. The new method, which involves just rotating raw materials with steel balls, was recently published in Nature Nanotechnology, a top journal in nanoscience.
Led by Professor Jong-Beom Baek in the School of Energy and Chemical Engineering and Professor Hankwon Lim of the Graduate School of Carbon Neutrality at UNIST, the research team announced that they have successfully created a mechanochemical process, capable of converting CO2 into CH4 efficiently at just 65°C. This simpler, low-energy approach could accelerate the shift toward a sustainable, carbon-neutral future.
The process uses a ball mill—a device containing small steel balls of a few millimeters in diameter—filled with catalysts and raw materials. As the mill rotates, collisions and friction activate the catalyst surfaces, enabling CO2 to be captured and react with hydrogen to produce methane.
Remarkably, the team achieved a 99.2% conversion rate of CO2 at this low temperature, with 98.8% of the reacted CO2 turning directly into methane, rather than byproducts. The process also proved highly effective in continuous operation, maintaining an 81.4% reaction participation rate and 98.8% methane selectivity even at 15°C, below room temperature. This demonstrates its potential for scalable industrial use.
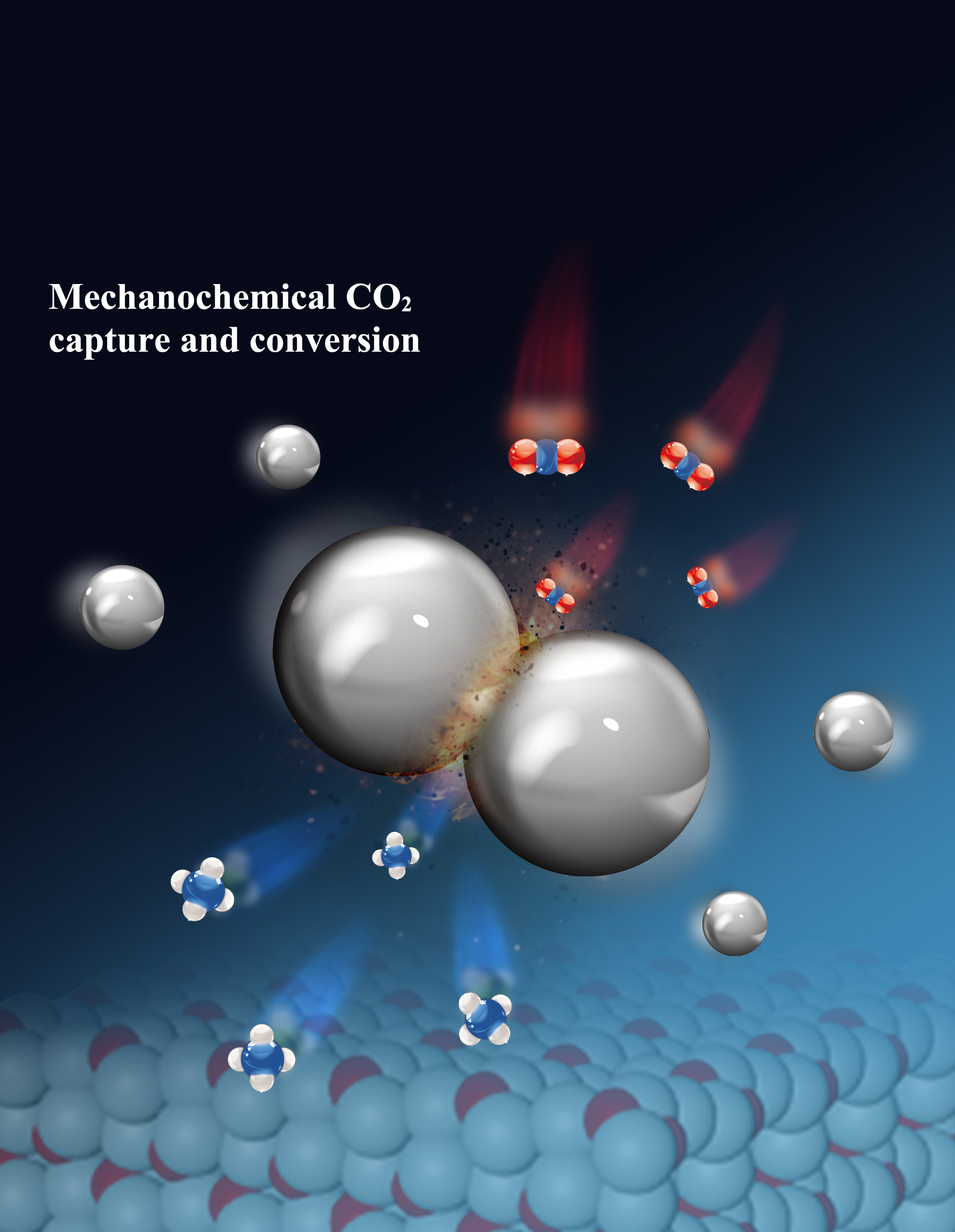
Schematic image, illustrating the mechanochemical CO2 capture and conversion.
The process uses commercially available zirconium oxide (ZrO2) and nickel catalysts, which are both affordable and widely used in industry. Nickel helps split hydrogen molecules, while zirconium oxide enhances CO2 activation. The mechanical impacts within the ball mill induce oxygen vacancies in zirconium oxide, trapping CO2 molecules and enabling their reaction with hydrogen on the nickel catalyst to produce methane.
Economic assessments suggest that because the process operates at low temperatures and utilizes readily available catalysts without extensive pre-treatment, it can significantly reduce equipment and operational costs. Professor Lim explained, “When powered by renewable energy sources, like wind or solar, this method could cut energy consumption in half compared to conventional thermochemical processes.”
Professor Baek highlighted the practical significance, “This technology makes it possible to convert CO2 directly into fuel on-site, without the need for high-temperature, high-pressure equipment. It not only reduces carbon emissions but also lowers infrastructure and transportation costs, offering a promising pathway toward carbon neutrality.”
This research was conducted in collaboration with Professor Qunxiang Li of the University of Science and Technology of China (USTC), with support from the National Research Foundation (NRF) of Korea and the UNIST Carbon Neutrality Demonstration and Research Center.
