The study is the first to report on the simultaneous absorption of carbon dioxide and water by a clay mineral at ambient concentrations of carbon dioxide. Their work, which earned them a 2024 R&D 100 Award and has a patent application in progress, was recently published in The Journal of Physical Chemistry C.
Cliff Johnston, professor of agronomy in the College of Agriculture and earth, atmospheric, and planetary sciences in the College of Science at Purdue University, led the study, along with undergraduate student Riley Welsh and research scientists at Sandia National Laboratories, who are co-authors of the recent study.
This research may expand the portfolio of absorbent materials for addressing one of the planet’s most challenging problems. Clays could be an inexpensive, accessible and abundant resource for absorbing carbon dioxide from the air and a powerful tool in addressing climate change.
Johnston, his research team at Purdue University and the Sandia National Laboratories team have been digging into what makes clay minerals tick for more than 30 years.
“Clay minerals have an exceptionally high surface area,” Johnston said. “One tablespoon of clay has approximately the same surface area as an American football field. Most of this surface area is found in the internal pores of the clay. Over decades of research, we have found that these internal pores have polar and nonpolar regions. Molecules like CO2 prefer the nonpolar regions, whereas water vapor prefers the polar regions. By selecting certain types of clay and manipulating their ionic structure, we can optimize for materials that can uptake CO2.”
The team studies a group of clays called smectites, which have large internal surface areas and are some of the most common naturally occurring nanomaterials on the planet. Both their abundance and their size make smectites promising candidates for large-scale environmental solutions.
Johnston’s team has a long history of exploring how smectites absorb toxic organic pollutants from water. Johnston has written nearly 200 papers, mostly covering how soil minerals interact with everything from pollutants to gases.
“Our prior work focused on absorption of toxic organic pollutants on clay minerals from aqueous solution, and we found that certain types of smectites have hydrophobic surfaces and can sorb significant levels of hydrophobic contaminants, such as 2,3,7,8-tetrachlorodibenzo-p-dioxin, one of the most toxic organic compounds known,” Johnston said. The main sources of dioxins are unintended byproducts of combustion and industrial manufacturing and are common contaminants found in Superfund sites.
Having laid a strong foundation, the team envisions advancing solutions to the urgent global challenge of carbon dioxide capture using widely available, affordable geosorbents.
In recent years, researchers worldwide have investigated clay-carbon dioxide interactions under extreme conditions, such as high temperatures and pressures, or through direct air capture using advanced materials like zeolites, mesoporous silica, metal-organic frameworks and metal-oxide-based adsorbents. For example, Climeworks’ Orca facility in Iceland uses unique solid amine-based sorbents to capture carbon dioxide from the air. However, clay minerals have largely been overlooked as promising sorbents until now.
The researchers focused on a specific smectite called saponite. They examined how saponite handles carbon dioxide and water vapor competing for space in the clay’s tiny internal pores. Unlike past studies that cranked up the heat to make clays absorb carbon dioxide, the researchers used humidity instead. They discovered that saponite exhibits a high affinity for carbon dioxide at low humidity levels, a finding they confirmed through advanced spectroscopic and gravimetric analysis.
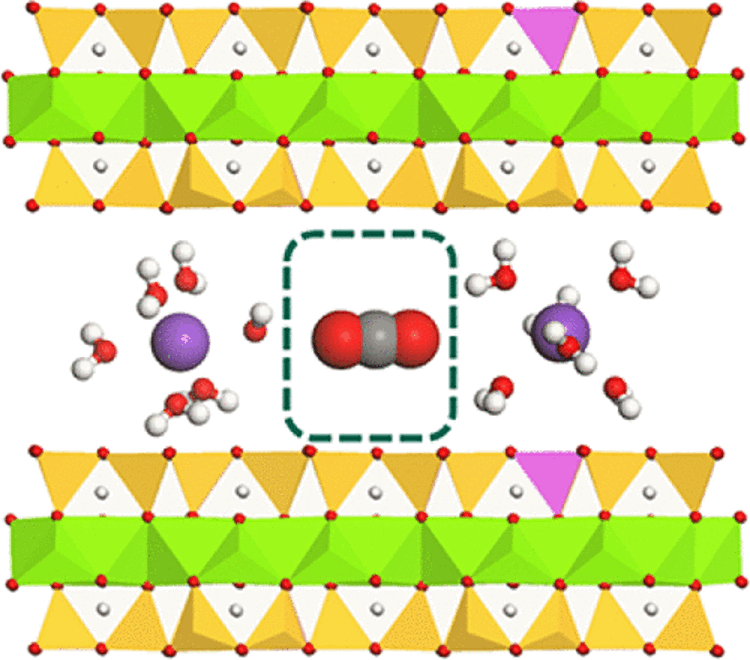
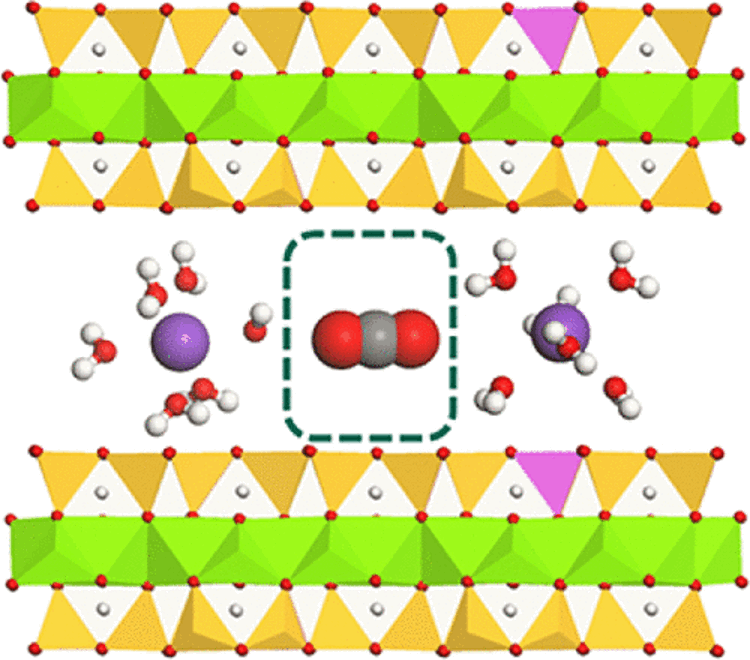
The clay interlayer sandwiched between two clay particles. The interlayer region is comprised of exchangeable cations (purple spheres), water molecules (red and white spheres) surrounding the exchangeable cations, and one carbon dioxide molecule (red and gray spheres) between the two hydrated cations. (Figure provided by Cliff Johnston)
The team’s research was supported by a Laboratory Directed Research & Development project at Sandia National Laboratories. Portions of the work were conducted at the Center for Integrated Nanotechnologies, a user facility operated for the U.S. Department of Energy Office of Science. The project also benefited from the robust strategic partnership between Purdue University and Sandia National Laboratories, which aims to address significant national challenges through collaborative research and development.